What Are the Elements That Touch the Zigzag Line Called
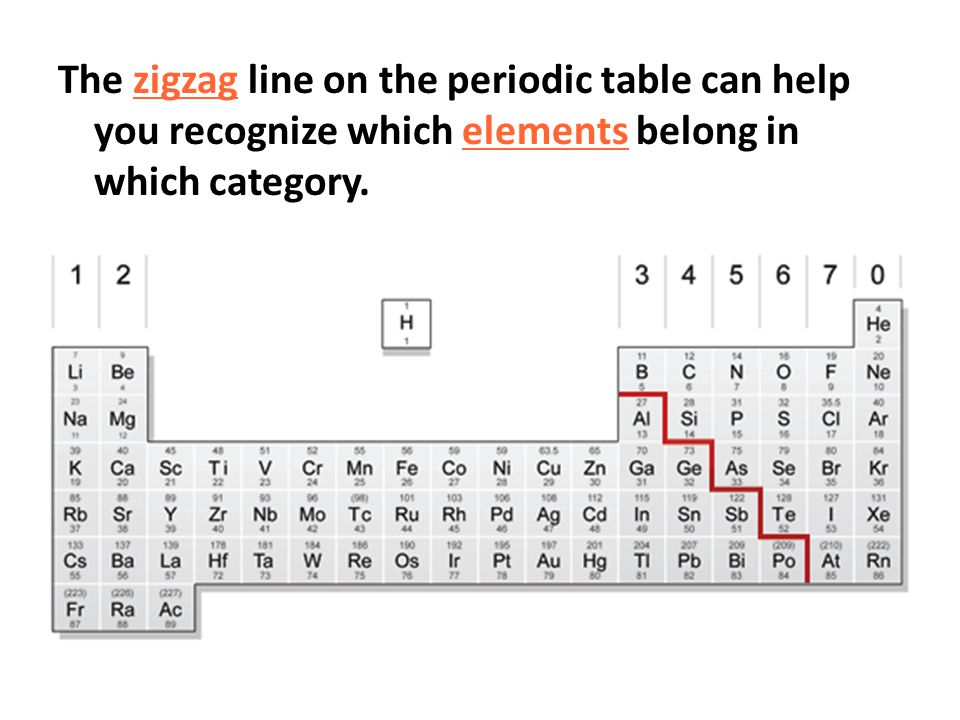
A small group whose members touch the zigzag line are called metalloids because they have both metallic and nonmetallic properties. The elements to the left of the zigzag line on the periodic table are called _____.

Solved The Zig Zag Line On The Periodic Table Separates
The elements in the middle of the table are called transition elements because they are changed from metallic properties to nonmetallic properties.
. The elements that touch the zigzag line are classified as metalloids. Elements in the second group have 2 outer shell electrons and are also very reactive. The elements that make up the zigzag line on the periodic table are called _____.
The elements that touch The zigzag line are classified as Comments आप यह पर elements gk touch question answers zigzag general knowledge line समनय जञन classified questions in hindi as notes in hindi pdf in hindi आद वषय पर अपन जवब द सकत ह. The zigzag line is called the zigzag line or is sometimes referred to as the Hays-McDaniel line Why is a line graph called a line graph. The elements in the middle of the table are called transition elements because they are changed from metallic properties to nonmetallic properties.
The elements that touch the zigzag line are classified as. Metals Most elements are metals. Most of the elements in the periodic table are classified as.
Color the square for Hydrogen pink. A small group whose members touch the zigzag line are called metalloids because they have both metallic and nonmetallic properties. In an element box the number at top is the Preview this quiz on Quizizz.
This is because every step you move down the group an extra shell of electrons is added along with a bunch of protons that increase nuclear attraction. The elements that touch the zigzag line are classified as. Elements in the first group have one outer shell electron and are extremely reactive.
Elements on the periodic table tend to follow this trend. Elements in the first group have. The horizontal rows on the periodic table are called Periods 3.
The elements in the far upper right corner are classified as. The elements that touch the zigzag line on the Periodic Table are called. Metallic character increases as you move down a group.
The elements that touch the zigzag line are classified as. - do not reflect light well. The elements that touch the zigzag line are classified as metalloids.
They are called _____ _____. Click here to get an answer to your question The elements that touch the zigzag line are classified as danyswrld danyswrld 09292020 Biology High School answered The elements that touch the zigzag line are classified as 1 See answer Advertisement. The elements on the right side of the periodic table are classified as nonmetals.
Place black dots in the squares of all alkali metals. Lightly color all metals yellow. Column in the Periodic Table are called.
Less metallic with the far right side of the table consisting of nonmetals. The elements in the middle of the table are called transition elements because they are changed from metallic properties to nonmetallic properties. Elements in the first group have 1 outer shell electron and.
The elements in the far upper right corner are classified as. Play this game to review Other. The elements in the far upper right corner are classified as.
The elements that touch the zigzag line are classified as 5. Nobal Gases or Non metals. Color each group on the table as follows.
The elements in the far upper right corner are classified as_____. Metals are found to the left of the zigzag line on the periodic table. Nonmetals Nonmetals are found to the right of the zigzag line on the periodic table.
The extra shell factor plays the dominant role making the. The elements that touch the zigzag line are classified as _____. Well the answer is quite simple.
They are called alkali metals. A line graph is called a line graph because first of all it uses lines and second of all its on a graph they use those lines to connect the dots so if you dont what im talking about with dots thats a whole. Elements in the first group have one outer shell electron and are extremely reactive.
Up to 24 cash back 4. The vertical columns on the periodic table are called around 2. The physical properties of these elements are.
Elements in the first group have one outer shell electron and are extremely reactive. Properties of the elements of the periodic table have a. The elements in the far upper right corner are classified as nonmetals.
A small group whose members touch the zigzag line are called metalloids because they have. Elements in the first group have one outer shell electron and are extremely reactive. A small number of elements touch the zigzag line are called metalloids or semi-metals because they have both metallic and nonmetallic properties.
The elements that touch the zigzag line are classified as. Metalloids Metalloids also called semiconductors are the elements that border the zigzag line on the periodic table. Most of the elements in the periodic table are classified as.
Elements in the first group Group 1A or IA of the periodic table are alkali metals such as hydrogen lithium sodium potassium rubidium cesium and francium. Most of the elements in the periodic table are classified as 4. The elements in the far upper right corner are classified as points Due by Frida Oct c 6.
Most of the elements in the periodic table are classified as.
What Is The Purpose Of The Zigzag Line On The Periodic Table Quora
0 Response to "What Are the Elements That Touch the Zigzag Line Called"
Post a Comment